CONCLUSIONS
Complete remission (CR) with standard-of-care HMAs in pts with intermediate-, high-, and very-high-risk MDS is usually infrequent and transient, and prognosis remains poor for these pts, who have limited second-line treatment options. Sabatolimab is a high-affinity, humanized anti-TIM3 IgG4 monoclonal antibody that blocks the TIM3 immune checkpoint receptor, which is preferentially expressed on leukemic stem cells and blasts and plays a key role in regulating adaptive and innate immune responses. In a phase Ib study, sabatolimab in combination with intravenous (IV) HMA was safe and well tolerated in pts with high-risk MDS with overall response rates of 61.1% and 57.9% with decitabine and azacitidine, respectively. We present an interim analysis of the STIMULUS-MDS US trial (NCT04878432) to assess the safety of sabatolimab in combination with not only parenteral but also oral HMAs in pts with intermediate-, high-, and very-high-risk MDS.
This is a multicenter, phase II, open-label, single-arm study of sabatolimab in combination with HMAs. Adults aged ≥18 years with intermediate-, high-, or very-high-risk MDS per Revised International Prognostic Scoring System criteria who were not previously treated with HMAs were eligible. Pts received an HMA of investigator's choice (decitabine/cedazuridine 35 mg/100 mg oral days 1-5, decitabine 20 mg/m 2 IV days 1-5, or azacitidine 75 mg/m 2 IV or subcutaneous [SC] days 1-7 or days 1-5 plus days 8 and 9) with sabatolimab 800 mg IV every 4 weeks starting at day 8 or any day between days 5 to 8. The primary objective of this study was to assess the safety of sabatolimab in combination with oral, IV, and SC HMAs. Secondary objectives included assessing the efficacy of the combination. The study includes a core phase for up to 12 months, an extension phase for efficacy and survival status up to 12 months after the core phase, and a post-treatment safety follow-up for 30 days following the last dose of HMA or 150 days following the last dose of sabatolimab, whichever is later.
This interim analysis included 33 pts with intermediate-, high-, and very-high-risk MDS at the data cutoff of 6 months after cycle 1 day 1 of treatment with sabatolimab and an HMA. The median age was 69 years (range, 39-86). At data cutoff, 8 pts were in ongoing treatment, and 25 pts had discontinued the core phase. Reasons for discontinuation included new therapy for study indication (n=6), physician decision (n=6), pt decision (n=5), progressive disease (n=4), unsatisfactory therapeutic effect (n=2), adverse event (AE) (n=1), and death (n=1). Of those who discontinued study treatment, 6 pts were in ongoing evaluation in the extension phase and 3 pts were in ongoing evaluation in the post-treatment follow-up phase. The median duration (range) of exposure was 3.9 (0.92-5.91) months for sabatolimab and 4.2 (1.05-6.08) months for HMAs.
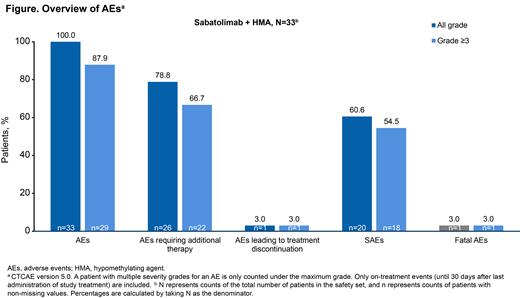
Among 33 pts evaluable for safety, most experienced all-grade and grade ≥3 AEs, AEs requiring additional therapy, and serious AEs (Figure). However, the most frequent (≥10%) grade ≥3 AEs were expected hematologic AEs, including febrile neutropenia (42.4%), anemia (39.4%), neutrophil count decrease (39.4%), and platelet count decrease (36.4%). Nonhematologic grades ≥3 AEs occurring in >1 pt included hypertension (18.2%), pneumonia (9.1%), hypoxia (6.1%), and fall (6.1%). Only 1 pt was reported with encephalopathy leading to treatment discontinuation, which was not attributed to study treatment. One pt had a fatal AE for which the cause was not identified but not thought to be related to study treatment by the investigator.
The best overall response results in up to 6 months of treatment were available for 22 pts. Marrow CR (mCR) with and without hematologic improvement (HI) was reported in 18.2% and 9.1%, respectively. Partial remission (PR) and stable disease (SD) with HI was reported in 4.5%, each. CR + mCR + PR was 31.8% (95% CI, 13.9%-54.9%), CR + PR + HI was 27.3% (95% CI, 10.7%-50.2%), and CR + mCR + PR + HI was 36.4% (95% CI, 17.2%-59.3%).
This interim analysis of the STIMULUS-MDS US study demonstrated that sabatolimab in combination with an oral, IV, or SC HMA was safe and well tolerated, with AEs consistent with previous reports for pts with MDS treated with HMAs. Response rates for the combination are preliminary, and more information will be provided with longer follow-ups.
Disclosures
Garcia-Manero:Bristol Myers Squibb: Other: Medical writing support, Research Funding; Genentech: Research Funding; AbbVie: Research Funding. Lyons:Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Exact Sciences: Research Funding; Astellas Pharma: Research Funding; Incyte Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Lessen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Texas Oncology: Current holder of stock options in a privately-held company; McKesson: Other: Leadership. Nandal:Novartis: Current Employment. Ashraf:Novartis: Current Employment, Current equity holder in publicly-traded company. Thellaboina:Novartis: Current Employment. Ruckel-Kumar:Novartis: Current Employment. Menssen:Novartis: Current Employment. Zeidan:Chiesi: Consultancy, Honoraria; Zentalis: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Schrödinger: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria; Mendus: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; BioCryst: Consultancy, Honoraria; Boehringer-Ingelheim: Consultancy, Honoraria; Taiho: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Geron: Consultancy, Honoraria; Foran: Consultancy, Research Funding; Otsuka: Consultancy, Honoraria; Tyme: Consultancy, Honoraria; Astex: Research Funding; Shattuck Labs: Research Funding; Amgen: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Notable: Consultancy, Honoraria; Syros: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Ionis: Consultancy, Honoraria; Orum: Consultancy, Honoraria; BeyondSpring: Consultancy, Honoraria; ALX Oncology: Consultancy, Honoraria; Lox Oncology: Consultancy, Honoraria; Syndax: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal